Safety of Research Chemistry Laboratory Chapter 2
Date: 2022-01-19 Source: RUANQI Classification: Resources
#This series is translated from the safety guidance book on research chemistry laboratory printed by the chemical safety committee of the Joint Council of American Chemical Societies. It is the best practical guide for freshmen and sophomores#
Chapter II your responsibility for laboratory safety
Introduction
Accident prevention is a collective responsibility that requires the full cooperation of everyone in the laboratory. You, your colleagues, teachers and colleges are responsible for the safety of the laboratory. Although everyone is responsible for the safety of the laboratory, the experimenter can prevent accidents most directly.
Accidents usually come from:
• indifferent attitude towards safety;
• failure to identify hazards or hazardous situations;
• failure to assess the risks involved in ongoing work;
• failure to be alert to the surrounding environment;
• failure to follow instructions or measures to minimize risks;
• fail to recognize the limitations of your knowledge and experience.
■ Incident and accident
Unexpected and undesirable events sometimes occur in the laboratory. In this booklet, we use the term "event" rather than "accident" to avoid implying that these events occur randomly or inevitably. Almost all incidents can be prevented if the guidelines and safety principles provided in this manual are followed.
You may be the victim of mistakes made by you or others. If you are doing incorrect laboratory operations and your classmate points out this, please be grateful. He or she may have just saved your life. If you find a classmate making a mistake, let him or her know. In addition, unsafe behavior should be reported to your instructor so that it will not happen again.

The guidelines in this chapter are designed to help you understand your role in maintaining the safety of the laboratory environment. Most of these guidelines and rules will apply to your introductory experimental course as an undergraduate. In some cases, when you enter more laboratory experiences, more information will be provided to guide you. At the end of this chapter, you will find a summary list of guidelines or safety rules. These guiding principles are the result of the application of many years of experience and lessons learned and are part of m in ramp: minimizing the risk of hazards. As you learn more about chemistry, you will learn to identify hazards (Chapter 3). But before entering the laboratory, you need to learn some basic knowledge about safety measures and safety equipment. Check safety measures frequently as a reminder.
■ In your future: minimize hazards and risks
In introductory and organic chemistry laboratory courses, substances are unlikely to be used under non environmental pressure, or high-energy materials that may react violently, such as foaming, overheating, boiling, or even small explosions under pressure (which are extremely rare). These types of laboratory experiments require the use of appropriate laboratory exhaust cabinets, workbench shields, chemical splashes and impact goggles to protect your eyes, and masks wide and long enough to protect your neck and ears.
Personal Protective Equipment
Personal protective equipment (commonly known as PPE) is one of the main ways to protect you from injury when working in the laboratory. It is important that you understand why your teacher requires you to use PPE.
PPE is used to eliminate or reduce some hazards encountered when working in the chemical laboratory. PPE includes items that protect specific parts of the body, such as eyes and hands. It usually includes gloves, goggles, laboratory coats and aprons. Don't rely solely on personal protective equipment to protect you, because it is often the last barrier between you and exposure.
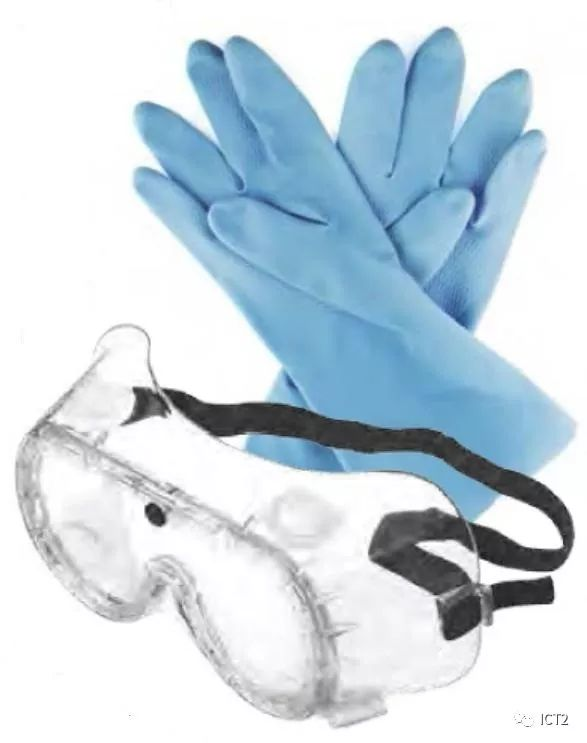
Hair and clothing (Lab attire)
Clothes worn in the laboratory should provide basic protection for your skin against splashing. Shorts, skirts and shirts expose your abdomen and will unnecessarily expose potential leaks from your skin. It is very important to minimize skin exposure in the laboratory environment. Heavy and loose clothes should not be worn in the laboratory. Loose sleeves may overturn laboratory items, drag over chemical spills, or pose a fire hazard in an open fire. Clothes should be made of natural fibers, such as cotton. Your mentor or college may require you to wear a lab coat or apron. Non combustible, non perforated aprons provide the most satisfactory and cheapest protection. If you are wearing a lab jacket or coat instead of an apron, it should have buttons, not buttons, so that it can be easily removed in case of contamination. In the lab, wear shoes (closed toe caps) with uppers made of leather or polymer leather substitutes that completely cover your feet and toes. This will provide the best protection for your feet against spills and falling objects. When you choose laboratory shoes, remember that the shoes you wear in the laboratory should not expose your toes, but should provide stability for standing and walking.
Long hair and loose clothes are strictly prohibited. Long hair is easily entangled by equipment, exposed to chemicals, or directly exposed to a lighted alcohol lamp. Jewelry such as rings, bracelets, necklaces and watches should be avoided in the laboratory. Jewelry may be damaged by chemical gases and vapors and spills. The chemical penetration between jewelry and skin will bring the corrosive into close contact with the skin and trap the chemicals there. Jewelry can also get entangled in equipment and cause injury.
Eye protection
Everyone in the laboratory, including visitors, must wear eye protection at all times, even without chemical operation. In some experiments, there is a risk of splashing, so it is necessary to wear indirect breathable goggles; For other experiments, safety glasses are enough. Since you may not want to buy two forms of eye protection, it is wise to use more protective glasses in various environments. Therefore, goggles to protect against chemical splashes are the preferred eye protection. The Chemistry Department of your college will assess the risk of hazards in your laboratory and determine the appropriate type of eye protection for experiments conducted in their academic laboratory. Ordinary types of glasses cannot provide appropriate laboratory eye protection against shrapnel from explosion or splashing of hazardous chemicals. Due to wearing normal glasses and no chemical splash goggles or safety glasses, serious injuries were caused.
Gloves
Gloves are an important part of personal protection. Your instructor will assess the risk of hazards, require the use of gloves when appropriate, and provide the appropriate type of gloves. Glove materials must be selected according to the chemicals used. Always check the gloves before each use to ensure that there are no cracks and holes. To avoid inadvertent dissemination of chemicals, remove gloves before leaving the work area and before handling items such as mobile phones, calculators, laptops, door handles, writing instruments, laboratory laptops and textbooks. You should wash your hands when you leave the laboratory, even if you wear gloves.

There are a variety of gloves and materials to choose from: neoprene, butyl rubber and many other materials. Different types of gloves have different glove lengths; Some cover the whole arm, some only cover the forearm, and some only cover the length of the wrist. People sensitive to latex should not wear gloves made of latex. Although cloth gloves or leather gloves can prevent hot or cold objects, do not rely on them to prevent dangerous chemicals. Instructors and colleges are responsible for assessing the risk of hazards and selecting appropriate gloves for specific applications.
Disposable gloves and gloves that have been penetrated by chemicals shall not be reused. Gloves cannot be safely reused because chemicals cannot be completely removed. Contaminated gloves are considered potentially hazardous waste, but this is not always the case. In any case, please put your used gloves in the designated hazardous waste container or dispose of them according to the instructions of your instructor.
■ Is proper glove material important? have an experienced lesson
In August 1996, Karen ▪ Dr. witthahn, a very successful researcher, is working on her current project in the laboratory, which requires the establishment of a standard to combine mercury compounds with proteins, and then study them by nuclear magnetic resonance (NMR) spectroscopy. The recommended binding compound is Dimethylmercury, which is a highly toxic compound. It is well known that it is a very toxic compound. Identify risks, Karen ▪ Dr. witthahn has tried many times to prepare the standard with less toxic mercuric chloride. When the results of these products were disappointing, she decided to continue to use Dimethylmercury to prepare the standard.
Karen ▪ Dr. vetha works in the laboratory exhaust cabinet, wears latex gloves (natural rubber) and adopts recognized prudent laboratory practice. During the transfer, two small drops of Dimethylmercury were dropped on her latex gloves. Unaware of the seriousness, she finished the day's work, cleaned up and did not report the incident.
Within one year, she developed severe symptoms and signs of acute mercury poisoning, and finally fell into a coma and died.
Her colleagues later tested the breakthrough time of Dimethylmercury on latex and found that it was 15 seconds or less. A lesson to be learned from this tragic event is to ensure that the gloves you choose have passed the manufacturer's test of the chemicals used and follow the manufacturer's recommendations - especially for a chemical whose error can be disastrous.
To read the full story, see "tribute to Karen witthahn". 1
■ In your future: the choice of gloves
Please note that gloves of any material cannot provide permanent protection. Eventually, the liquid will penetrate into all glove materials. The material of the gloves is rated by the manufacturer using the breakthrough time (the time required for the specific chemicals in contact with the gloves to pass through the gloves). For many organic solvents, the breakthrough time can only be a few minutes. Since the air permeability of gloves made of the same or similar materials may vary from manufacturer to manufacturer, please refer to the information provided by the glove manufacturer for specific guidance. If a chemical diffuses through gloves, it will cling to your skin; You may get more exposure than you don't wear gloves at all. More information is available from the glove manufacturer. Online search for "chemical glove selection" will produce some useful information websites.
Laboratory Protocols
Laboratory environment
The chemistry laboratory can provide rich learning opportunities, but when working in the laboratory, you should be vigilant about your behavior and the behavior of the people around you. Changes in procedures, including changes in the chemicals used or the quantities to be used, may be dangerous. Changes can only be made with your teacher's knowledge and approval.
Pay attention to the surrounding environment before working in the laboratory. Determine the location of exits, fire alarm stations, eye washers, safety showers, fire blankets, first aid boxes and fire extinguishers; Practice walking to the exit. This is part of P in ramp: emergency preparedness.
Do not eat or drink in the laboratory to ensure that there are no pollutants that may lead to the intake of laboratory chemicals. Food or beverages shall not be brought into or stored in the laboratory.
Laboratory visitors
All laboratory visitors, no matter how short their visit time, should wear eye protection. Visitors, such as friends and relatives, may not be aware of the danger and may inadvertently act unsafe. Before taking visitors into the laboratory, please obtain the approval of the laboratory instructor.
Room arrangement
In laboratories and other places, keeping things clean and tidy is usually a safer environment. Keep aisles and access to safety equipment free of obstacles, such as chairs, boxes, open drawers, backpacks and waste containers. Avoid the risk of slipping and keep the floor free of spilled liquid, ice, plugs, glass beads or rods, and other similar small objects. Keep the workplace and storage area away from broken glassware, residual chemicals and dirty glassware. Broken glassware should always be disposed of in broken glass containers, not in ordinary dustbins. If glass breaks or chemicals spill, please inform your instructor immediately. Dispose of chemical waste and unused chemicals according to the procedures required by the laboratory. Before leaving the laboratory, wipe the workbench area to avoid inadvertent contact with chemical residues. Do not leave chemicals on the balance because it may unnecessarily expose the next user to chemicals; In addition, electronic scales are expensive and easily damaged by corrosive chemicals.

■In your future: special cleaners
It is reported that many events involve strong oxidizing cleaning solutions, such as nitric acid or chromium sulfuric acid mixtures. Do not use flammable solvents as cleaners unless specifically requested by the instructor. Do not use strong cleaners such as nitric acid, chromic acid, sulfuric acid or other strong oxidants unless specifically instructed to use them, and then only after special training and appropriate protective equipment.
Labeled chemicals
Incorrect or inadequate labeling of chemical containers leads to many adverse events. Labels usually refer to "manufacturer's label" and "secondary label". It is important that the manufacturer's label is not changed, overwritten or otherwise changed until the container is verified to be empty. Usually, empty containers will be reused, for example, with solutions prepared by students. Before reusing the container for another solution, the discarded label shall be completely removed, and the container shall be thoroughly cleaned and air dried. Writing an existing manufacturer's label with a tag is not acceptable. Under no circumstances should the container have two labels, each on both sides of the bottle.
Although you are unlikely to participate in the management of the manufacturer's container, you can prepare the solution and store it in the drawer of the guided and organic chemistry laboratory. The secondary label used temporarily (during the laboratory or until the future laboratory) shall at least have the name of the chemical, the name of the person who filled the container, the date when the container was filled and the hazard. Containers prepared for long-term storage shall be labeled in accordance with the globally harmonized system of classification and labelling of chemicals (GHS) (see Chapter 3).
Cleaning glassware
Clean dirty glassware in the laboratory sink with hot water, environmentally acceptable detergent and brush of appropriate hardness and size. Don't brush glassware with a brush. When washing dishes, be sure to wear chemical splash goggles and gloves, if necessary. Many laboratory faucets have serrated nozzles that produce high-speed water flow. When using these types of faucets, be sure to adjust the water flow slowly and do not put glassware under them. The water will flow out strongly, splash back on your face, or knock the glassware off your hand. Avoid accumulating too many items in the clean area.
The working space around the sink is usually limited, and stacking dirty or cleaned glassware can lead to cracking. Remember that the muddy water in the sink may hide the sharp serrated edge of a broken glassware, which is intact when put into the water. If the glassware in the sink is broken, drain the water. Use a pair of anti cutting gloves, tweezers or pliers to remove broken glass. Special care should be taken when cleaning the drainage area because glass fragments can get stuck in the hole and are almost impossible to find. In order to minimize the damage of glassware, there may be rubber or plastic pads at the bottom of the sink, which will not block the drainage pipe.
Inhalation of harmful chemicals
If you are asked to smell something in the laboratory, blow the steam into your face with your hand and smell it gently. You shouldn't put your nose directly on a chemical container to smell chemicals. The presence of smell is not a reliable indicator of potential harm, while the absence of smell is not a reliable indicator of no harm.
Some people think that if they can smell a chemical, it will hurt them. This is not necessarily true. If you smell a chemical and you're inhaling it, of course it's right. Some harmful chemicals are odorless, while others can paralyze the sense of smell. The concentration of some chemicals is harmful and cannot be detected by human nose; Some chemicals, even if they have an obvious smell, are not harmful by inhalation.
Many substances that may or may not have an odor are harmful if their vapors, dust or fog are inhaled. The label on the container and the safety data sheet (SDS) of the chemical (see Chapter 3) may carry a warning of inhalation hazards. Your instructor will guide you in the distribution and handling of these substances in the laboratory.

Disposal of chemicals
Correct treatment of reaction by-products, residues, waste chemicals and contaminated substances is an important factor to prevent accidents, and there are very strict regulations on the treatment of chemicals. Improper disposal may cause serious damage to the environment or bring legal problems to your college. It is the responsibility of each student to ensure that these wastes are treated in a manner that minimizes personal hazards and recognizes the possibility of environmental pollution.
Usually, your reaction by-products and residual chemicals will be poured into properly labeled waste or hazardous waste containers for proper disposal. Your instructor will instruct you to use designated, labeled waste containers. Most likely, different types of chemicals will use different containers. Dispose of your waste according to the specific methods specified by the instructor. Pouring waste into the wrong container may cause unexpected adverse reactions, resulting in fire or explosion (see Chapter 3 "containing nitric acid - do not add organic solvents"). Remember to pay attention and follow the instructions.
Sometimes, your reaction by-products can be neutralized or deactivated as part of your process, which helps to reduce waste treatment and reduce treatment costs. Once your by-products are removed from the experiment, they will be subject to hazardous waste regulations established by your state government and the Federal Environmental Protection Agency (EPA).

■Some conventional disposal guidelines are as follows
• When handling chemicals, each type of chemical waste should be placed in specially labeled containers. Read the label carefully and replace the bottle cap after use.
• Never put chemicals into a sink or drain unless your teacher tells you that local regulations allow them to enter the sewer sanitation system. For example, water and dilute aqueous solutions of sodium chloride, sugar and soap from the chemical laboratory can be treated in the washing tank.
• Put ordinary waste paper into a wastebasket separated from chemical waste. Materials contaminated with chemicals, such as paper towels used to clean up spills, may need to be placed in a special container and marked for use. Your instructor will tell you whether you need to collect cleaning materials for hazardous waste or put them into containers in landfills.
• Broken glass should be placed in marked waste containers. If broken glass is contaminated with chemicals, ask your teacher where to put the glass. Your college may still use mercury thermometers, but most thermometers have been replaced by thermometers for alcoholic liquids. If you happen to use a mercury thermometer and it breaks down, inform your laboratory instructor immediately. Leaking mercury requires special cleaning procedures and should not be ignored because Mercury is toxic. Broken thermometer fragments may contain mercury; Broken glass contaminated with mercury shall be placed in labeled containers.
Summary
Most of what we know about science is learned in a laboratory somewhere. Laboratories can be interesting places to learn, but they can also be places for hazardous chemicals and equipment. In order to protect yourself and your peers, it is important to be aware of these dangers and risks and avoid actions that may lead to events that may harm you and your classmates or damage the laboratory. As a student, your responsibilities include preventing accidents in the chemical laboratory. The following list summarizes the basic guidelines designed to help you accomplish this important responsibility (to minimize the risk of harm). When you're in the lab:
Proper behavior
• don't work alone.
• do not conduct experiments or change procedures without approval.
• maintain awareness of the surrounding environment and purposefully interact with others.
• do not remove chemicals from the laboratory without proper authorization, and report any observed unauthorized removal of chemicals by others to your instructor.
• don't play tricks or fool around in the chemistry laboratory.
• if you find a violation of laboratory safety rules, please inform your instructor; You can save people's lives.
Appropriate laboratory clothing
• prevent skin exposure by covering the skin.
• the feet must be completely covered, and the skin must not be exposed between the top of the shoes and the bottom of the skirt or trousers.
• long hair is strictly prohibited. Avoid wearing loose clothes and taking off scarves and jewelry.
Safe disposal of chemicals
• read the experimental procedures in advance, listen carefully to the guidance of the tutor, and indicate the safety requirements of the experiment in the preparation Notes before the experiment.
• do not smell chemicals directly. When you need to smell something, blow the steam to your face with your hand and smell it gently.
• when the reagent is removed, do not put it back into the original container
Safe operation of equipment
• do not suck with your mouth. Be sure to use a straw or a suction ball.
• do not use exposed or worn hot plates.
• check Bunsen burner hose for holes.
• always ensure that the load of the test tube in the centrifuge is balanced.
Engineering controls and personal protective equipment
• when working in the laboratory, be sure to wear correct eye protection. Your instructor will tell you the level of eye care you need.
• if required, wear chemical resistant laboratory overalls or aprons.
• work in the laboratory fume hood as directed.

Proper housekeeping
• minimize the risk of tripping and keep the aisle away from backpacks and other tripping hazards.
• keep chemicals and instruments away from the edge of the laboratory bench or other work space to prevent leakage.
• dispose of chemical hazardous waste according to the instructions. If you are unsure, please always seek guidance.
• wash lab coats or other clothes frequently. The chemicals on these clothes are separate from personal clothes.
• clear the workspace for the next user.
• clean spills on the balance as directed.
Proper hygiene
• do not prepare or store (even temporarily) food or beverages in the chemistry laboratory.
• do not eat any food or drink in the chemistry laboratory.
• do not wear or carry laboratory aprons or laboratory coats into food eating areas.
• Please do not chew gum, smoke, use cosmetics or lipstick in the laboratory.
• Note that unpacked cosmetics, food and tobacco products will absorb chemical gases.
• do not put your hand or pen on your face or mouth when working in the laboratory.
• do not touch contact lenses in the laboratory unless they are removed in an emergency when an eye washer or safety shower is required.
• before leaving the laboratory, be sure to wash your hands and arms with soap and water, even if you wear gloves.

Emergency preparedness
• be fully familiar with the location and use of safety equipment and facilities, such as exits, evacuation routes, safety showers, eye washers, fire extinguishers and leakage tools.
Reference resources:
1 Dartmouth Toxic Metals Superfund Research Program. A Tribute to Karen Wetterhahn.
www.dartmouth. edu/~toxmetal/about/tribute-to-karen-wetterhahn. html (accessed March 6, 2017).




