Safety of Research Chemistry Laboratory Chapter 3 (2)
Date: 2022-01-19 Source: RUANQI Classification: Resources
#This series is translated from the safety guidance book on research chemistry laboratory printed by the chemical safety committee of the Joint Council of American Chemical Societies. It is the best practical guide for freshmen and sophomores#
Chapter III guidelines for chemical hazards
Flammability
Solvent
A solvent is a liquid used to dissolve or disperse other reagents. Organic solvents represent a large class of liquids that you will be exposed to, especially in organic chemistry laboratories. Many organic solvents have obvious flammability hazards. Flammable solvents such as acetone, n-hexane, methanol, ethanol and acetonitrile are commonly used in chemistry teaching and scientific research laboratories.

Organic solvents can be divided into three categories: one containing only hydrogen and carbon (hydrocarbons), one containing both oxygen (oxygenated solvents), and one containing halogen (halogenated solvents). Many (but not all) halogenated solvents (such as dichloromethane, carbon tetrachloride and chloroform) are nonflammable but highly toxic. Hydrocarbons (such as hexane, toluene and xylene) and oxygenated organic solvents (such as methanol, ether and acetone) are usually very flammable. Do not rely on generalizations about Flammability; Be sure to check the label on the solvent you use because it will show flammability.
It is important to understand that flammable liquids themselves cannot burn; What burns is the vapor in the liquid (the gaseous state of chemicals). The rate of combustible gas produced by liquid depends on its vaporization rate, which increases with the increase of temperature. Therefore, flammable liquids are more dangerous at high temperature than at room temperature. Many organic solvent vapors are denser than air and can reach the ignition source and "flash back". Therefore, please remember that when you pour out the combustible solvent, you are also pouring out the invisible combustible gas. If these gases are exposed to a nearby fire source, it will cause a fire.
All flammable liquids and solids must be kept away from oxidants and accidental fire sources, such as hot plates in fume hoods. Do not store solvents in the fume hood where you work. Flammable organic solvents shall be stored in flammable cabinets at room temperature unless other storage conditions are indicated on the manufacturer's label.
Flammable solid
Unlike Pyrophoric solids (discussed in the reactivity section), combustible solids require a source of ignition. Many metals commonly used in teaching laboratories, such as iron, magnesium, calcium and aluminum, are flammable. The finer the material is divided, the greater the risk. Flammable metal fires are easy to be extinguished, and special fire extinguishing materials are required. Do not put fine metal into the dustbin containing flammable materials. In fact, you shouldn't put any chemicals in the dustbin.
Corrosive
1. Corrosive substances
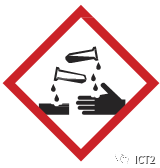
Corrosion is the gradual destruction caused by the action of a chemical substance on metals or organisms. All strong acids (such as hydrochloric acid, sulfuric acid and nitric acid) and all strong bases (such as hydrochloric acid, sulfuric acid and nitric acid). Some weak acids (such as acetic acid, carbonic acid and phosphoric acid), some weak bases (such as ammonium hydroxide) and some slightly soluble bases (such as calcium hydroxide) are corrosive.
■ concentration and dilution
In chemistry, "thick" and "thin" refer to the special properties of acids.
Aqueous solutions of acids are usually produced as a percentage of the weight of the acid. For example, concentrated hydrochloric acid (AQ) is 37% (w / W) or 12 M. Concentrated sulfuric acid (H2SO4) is 96% (w / W) or 18 M.
Concentrated acid solutions may be diluted to any given lower concentration, and the term "dilute" may refer to any such solution. Therefore, local practice may refer to a solution of 6m, 1m or < 1m as "dilution".
When chemists talk about strong and weak acids, they mean the dissociation of protons in solution. Strong acids decompose 100%, while weak acids do not.
"Strong" is not equal to "thick" and "weak" is not equal to "thin". Whether concentrated or dilute, strong or weak, most acids have the ability to damage tissues, which depends on their pH value, exposure time and acid protein binding ability.
Even acute exposure to corrosive chemicals can irreversibly destroy life. Your eyes are particularly vulnerable. The higher the acid or alkali concentration, the longer the contact time and the greater the damage. Some acids and bases can cause damage within 15 seconds of contact. Therefore, you should always wear chemical splash goggles when handling corrosive substances. Chemical laboratories using corrosive substances need to have an eye wash and safety shower, which can be accessed from any place in 10 seconds. You should know where the eye wash / shower is, and then practice walking there, maybe close your eyes.
In your first chemistry laboratory, the corrosive solutions you are most likely to encounter at different concentrations are hydrochloric acid, sulfuric acid, phosphoric acid, acetic acid, nitric acid, sodium hydroxide and ammonium hydroxide.
2. Sour
When diluting the concentrated acid solution, remember to slowly add the acid to the water when stirring, because the heat of the solution will greatly increase the temperature. For example, the heat of the solution generated during the dilution of concentrated sulfuric acid is so large that it is usually carried out when the beaker freezes to prevent the solution from boiling and splashing.
In addition to corrosivity, acids commonly used in many laboratories have other hazardous properties. All aqueous solutions of hydrogen halides (HF, HCl, HBr and HI) are toxic, but HF requires special attention (see "future: particularly dangerous acids"). These acid vapors are serious respiratory irritants.

Concentrated sulfuric acid is a very strong dehydrating agent (capable of removing water). Except for very dilute solutions, all solutions can be oxidized (see "reactivity" section). Phosphoric acid is a weak acid. Concentrated acid is a viscous liquid. Like sulfuric acid, it is a strong dehydrating agent.
Nitric acid is also a strong oxidant. It usually reacts faster than sulfuric acid. If diluted nitric acid comes into contact with the skin and is not completely washed off, it will cause the exposed skin to turn yellowish brown because of the protein denaturation reaction. Nitric acid is further discussed in the section "reactivity".
■ In your future: acid can be extremely dangerous
Perchloric acid, picric acid, or hydrofluoric acid (HF) are unlikely to be encountered in your first chemistry laboratory. Each acid has many serious hazards. Their use in academic research laboratories is not as common as before, but their use and storage must be carefully controlled when they are needed. The harmful consequences of improper use of these reagents are enormous. Perchloric acid and picric acid are also discussed in the "reactivity" section.
HF is toxic. It is rapidly absorbed through the skin, goes deep into the skin and destroys subcutaneous tissue. Contact with diluted HF solution is usually painless for several hours, followed by severe burns, adverse internal effects (including bone destruction) and severe pain. 25% of people will die when exposed to this acid. Do not deal with HF without a full understanding of the hazards of HF, very specific training and appropriate personal protective equipment (PPE) and ensuring that emergency procedures are in place.
3. Alkali
Alkali metal hydroxides and aqueous ammonia solutions are most commonly used in academic laboratories. Sodium hydroxide and potassium hydroxide are strong alkalis, which have great destructive effects on skin and eyes. Ammonia in aqueous solution, commonly known as ammonium hydroxide, is a weak base. Ammonia vapor is highly irritating and toxic. Ammonia is particularly harmful to eyes.
Strong alkalis are corrosive and can cause serious destructive chemical burns. If splashed into the eyes, they can lead to blindness. Bases do have a good warning effect: they usually have a smooth feeling, because the oil on your skin will saponify, so you know to rinse until that feeling disappears. However, if not completely removed by flushing, the strong alkali solution may not cause pain until the corrosion damage is quite serious.
Reactivity
Chemicals have the ability to react with other chemicals and convert them into new substances. This is the basis of all chemical experiments. Reaction itself is not necessarily a problem, but uncontrolled reaction is a big problem. In order to manage reactivity, you must learn to recognize certain characteristics of chemicals. Not all reactions are based on the interaction of chemicals. Some chemicals are self reactive, while others are unstable and will decompose violently if disturbed. Reactivity includes all these characteristics. As described in the introduction to this chapter, even harmless chemicals (such as water) can cause harm where appropriate.
■ In your future: chemical incompatibility and safe storage of chemicals
As you learned in this chapter, chemicals have hazardous properties that must be managed during use and storage. Most experiments in chemistry laboratories use chemicals that react with each other, and these chemical reactions are useful and sometimes harmful. Reaction between acid and base; Oxidant reacts with reductant. When these reactions present specific risks, we usually call the two reactants "incompatible". Depending on the degree of incompatibility, the reaction may be mild or severe. Many of these incompatibilities have been documented in the literature and on the Internet. As long as you search the "chemical incompatibility diagram", you can easily find Internet resources. A list of other published resources is given in the appendix.
The concept of incompatibility also plays an important role in studying how chemicals are stored in laboratories or warehouses. A person's first tendency is to store chemicals alphabetically or experimentally, but this may lead to incompatible chemicals being stored adjacent to each other. If the container leaks or breaks, chemicals mix and react, there will be a potentially dangerous situation. The chemical storage system isolates different kinds of chemical substances to keep incompatible chemicals well isolated from each other. Similarly, a large number of resources on this subject can be found in print and online.
■ "contains nitric acid - do not add organic solvents“
We can't just talk about reactive chemicals without talking about nitric acid. You are likely to use this acid in your first chemistry laboratory.
Many events related to nitric acid occur in academic laboratories every year. Most of them are caused by the high-energy reaction between nitric acid and ordinary organic substances (such as ethanol and acetone).
Do not mix nitric acid with organic solvents. Most of the reported incidents involve incorrectly labeled waste containers or users who are unaware of this hazard and mix the two in one waste container, which is a recipe for disaster. Do not use organic cat litter to clean up nitric acid spills.
Oxidant
Even in your first chemistry lab, you may encounter a class of reactive chemicals that are classified as oxidants. These substances can provide oxygen in the reaction or can be reduced (to obtain electrons), thereby promoting the oxidation of another substance (loss of electrons). When you study chemistry, you will be asked to learn nomenclature, the language of chemistry. The names of these chemicals end with "ate", "ite" or "IC" or begin with "per". This is only a broad description, not inevitable.
Some common oxidation solutions you may encounter in your first experiment are nitrate, potassium permanganate, hydrogen peroxide, bromine (in the organic chemistry laboratory), sulfuric acid and various metal salts of nitric acid. Of particular relevance to oxidants is proper storage. They shall be stored separately from any oxidizable chemicals or materials. This includes not putting bottles on wooden shelves or with flammable solvents.
Formation of peroxide solvent
Hydrogen peroxide is a compound composed of two oxygen atoms connected by a single bond (- O - O -). You may be familiar with the most common of these compounds: hydrogen peroxide. In organic peroxides, one or two hydrogen atoms are replaced by an organic group. All peroxides are reactive, but this is especially true for organic peroxides because they cause unusual stability problems. In your first experiment, you are unlikely to encounter this dangerous chemical, but you may use a solvent that will form unstable peroxides after exposure to air or light for a period of time.
A few organic solvents (such as ethers and some non aromatic unsaturated cyclic hydrocarbons) can form potentially explosive peroxides and peroxides. These solvents are particularly dangerous if they evaporate in a near dry state. You can use ether in your organic chemistry laboratory, and your teaching laboratory manager should ensure that the solvent is peroxide free. However, if you have any questions, ask your mentor.
In any case, do not move or open the old container of organic solvents until you know which organic solvents will form peroxides. All organic peroxides are extremely flammable and some are explosive. Hydrogen peroxide, which exists as a polluting reagent in the solvent, can change the process of planned reaction ions. If you see crystals around the bottle cap or in organic solvents, please inform your mentor immediately.
■ In your future: particularly dangerous hazards
As mentioned in the section "corrosivity", some less commonly used acids, such as those listed here, are also reactive hazards.
Perchloric acid is a very strong high-temperature oxidant, which can react explosively with organic compounds and other reducing agents. Digestion of samples with this acid requires special equipment and training.
Picric acid is a solid acid whose chemical structure is similar to trinitrotoluene (TNT). This acid must always be kept moist because dry picric acid is explosive. If you encounter a bottle of this dangerous solid and cannot determine the state or age, seek the help of your instructor or consultant immediately. This is not a chemical that untrained students can handle.
Pyrophoric materials are very reactive reagents, and even small amounts are dangerous because they are easy to ignite (in 5 minutes or less) after contact with air. These compounds require special training before use. Examples of these compounds are organic stone compounds (such as tert butyl sulfur solution) and some very fine metal powders (such as magnesium). These substances can only be used under the supervision of documented senior training and consultants. These compounds will have the GHS hazard code h250 (see the globally harmonized system of classification and labelling of chemicals).
Shock sensitive materials and friction sensitive materials are not specifically discussed as a separate category, but some materials are mentioned in the "reactivity" section. Some famous chemicals in this category (broadly speaking) are metal azides, perchlorates and organic peroxides. The use of these materials shall be controlled as described above.
Recognizing Chemical Hazards: Sources Of Information
Your mentor or consultant
The instructor of each laboratory is an important source of chemical safety information. As the starting point of laboratory exposure, the instructor is ready to explain to you the hazards related to laboratory chemicals in use and provide you with necessary preventive measures to reduce risks and prevent exposure.
Globally harmonized system of classification and labelling of chemicals (GHS)
Ghs5 is about communicating hazards to users; Remember R and a of ramp: recognize hazards and assess the risks of hazards. Using GHS for hazard identification requires you to have a basic understanding of the elements of the system. In GHS, there are 17 physical hazards, 10 health hazards and 2 environmental hazards. In each category, hazards are placed in one category according to various criteria specific to the classification. Each category is assigned a number or a letter, such as 1 to 5 or a to E. In GHS, the lower the category value of each chemical, the more serious the harm is.
Hazard categories communicate with users through pictograms, hazard instructions, prevention instructions and signal words. Hazard categories are particularly helpful in assessing the relative risk of hazards. For example, acetonitrile is classified as flammable liquid (Class 2) in GHS, and its flame pictogram and signal word "danger". The solvent is also listed as four highly toxic chemicals by oral fish eggs. Remember that category 1 or category A is the most dangerous level in this category.
The occupational safety and Health Administration (OSHA) defines chemical substances with physical hazards as "chemical substances with one of the following hazardous effects: explosives; flammable (gas, aerosol, liquid or solid); oxidant (liquid, solid or gas); reaction; spontaneous combustion (gas, liquid or solid) ; Automatic heating; Organic peroxide; Metal corrosion; Gas pressure; Contact with water releases flammable gases; Or combustible dust.
The occupational safety and health administration defines chemicals hazardous to health as "chemicals that can cause one of the following dangerous effects: acute toxicity (any route of exposure); skin corrosion or irritation; serious damage to or irritation to eyes; respiratory or skin sensitivity; germ cell induction; carcinogenicity; reproductive toxicity; toxicity of specific target organs (single or repeated exposure) ; Or inhaling hazardous substances. "
GHS icons and hazards
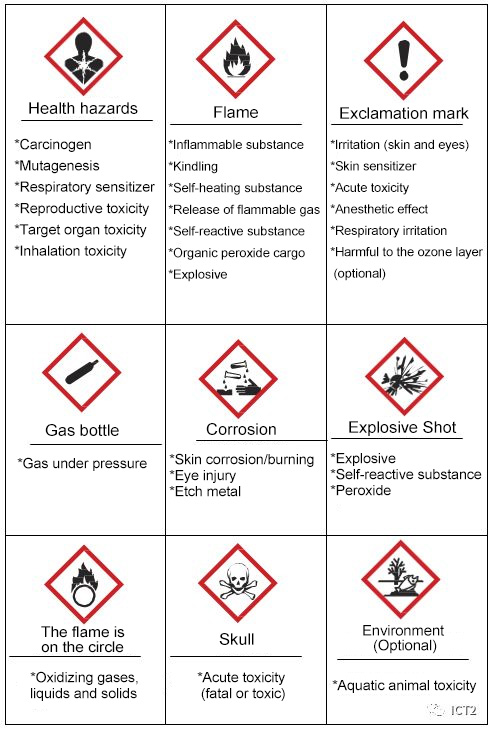
GHS elements
An icon is a picture representing a concept. GHS uses nine pictograms to visually remind users of the hazard level of the chemical. The GHS pictogram and the hazard categories it covers are shown in Figure 1. The manufacturer must evaluate each hazard level of each type of chemical. If the degree of harm in a class is large enough, the pictogram needs to be used in the label and SDS. There is no minimum or maximum number of pictographs that can be guaranteed by the material.
A hazard statement is a short statement describing each physical, health and / or environmental hazard. There are quite a number of dangerous statements, and each statement has an H code as an alphanumeric identifier.
A precautionary statement is a short statement indicating how to handle, store, prevent contact and dispose of a substance. There are even more precautionary statements than danger statements. Each is assigned a P code as an alphanumeric identifier.
Signal words provide users with an immediate indication of the severity of hazards in each class. There are two signal words: "danger" and "warning". In a specific hazard category, "danger" is used for more serious hazards and "warning" is used for less serious hazards.
■ In your future: understand the globally harmonized system of classification and labelling of chemicals
How can you know all the dangers and precautionary statements? The short answer to this question is that you do not need to remember these codes because the text of the hazard statement or prevention statement requires information related to hazardous chemicals. However, if you happen to see an H code or a P code somewhere, there is at least one quick way to identify the basic classification represented.
All hazard codes begin with the letter h followed by three digits. The first number after H indicates whether the hazard is physical (2), health (3) or environment (4). You can also learn to identify groups in classes. For example, codes 220 to 230 represent some type of flammable hazard.
All prevention statements begin with the letter P, followed by three digits. Here, the first number after P represents generality (1), prevention (2), response (3), storage (4), or processing (5).
Manufacturer's container label

The last barrier between hazard identification and users is at your fingertips: the label on the container where chemicals are stored. This is why OSHA's hazard communication standard (29cfr1910.1200, Appendix C) is consistent with GHS and requires labels placed on hazardous chemicals to comply with the standard format. As of June 1, 2015, hazardous chemicals are required to have specific label elements required by this standard.
Product identifier. This is usually the name of the International Union of pure and Applied Chemistry (IUPAC), but it can also be replaced by the trade name or common name of the chemical. CAS registration numbers are almost always included. The identifier on the label must exactly match the identifier in the SDS (Section 1).
1) Supplier information. This includes the name, address and telephone number of the manufacturer, importer or responsible party.
2) Signal word. If necessary, signal words are determined by the degree of danger of any category. Although a chemical may have multiple hazards and need a signal word, only the maximum hazard will appear on the label.
3) Each hazard statement, such as text. Unless otherwise specified in the regulations, it must be indicated on the label.
4) All pictograms are determined by the degree of danger.
5) Each precautionary statement, such as text.
6) The label may also include supplementary hazard information determined by the manufacturer.
Safety data sheets (SDSS)
According to GHS, OSHA defines hazardous chemicals as "any chemicals classified as physical or health hazards, simple asphyxiants, combustible dust, spontaneous combustion gas or other unclassified hazards".
SDS of hazardous chemicals is a document describing the hazards of the chemical and the preventive measures that must be taken to avoid hazards. The occupational safety and safety administration requires employers to keep a "safety standard specification" for each hazardous chemical in the premises for the inspection of employees who need it. As a student, you can also apply for chemicals from SDS. The Internet also makes it very easy and educational to search and find SDS on the Internet.
GHS SDS is divided into 16 parts. The order of information displayed in SDS is regulated by regulations, so the information given by manufacturers is relatively uniform. SDS must be in English. "Design of safety data sheet" shows the summary of 16 parts that must be included in SDS.
■ Design of safety data
The following is a summary of the information that the manufacturer must provide in the safety data sheet 6. OSHA has prepared a summary detailing each section.
Section 1: identification
Section 2: hazard identification
Section 3: ingredients / ingredient information
Section 4: first aid measures
Section 5: fire protection measures
Section 6: accidental release measures
Section 7: handling and storage
Section 8: exposure control / personal protection
Section 9: physical and chemical properties
Section 10: stability and reactivity
Section 11: toxicological data
Section 12: ecological information
Section 13: disposal considerations
Section 14: Transport Information
Section 15: regulatory information
Section 16: other information.
■ In your future: toxicology and regulatory provisions
Exposure guidelines for many chemicals have been developed and will be included in SDS. Guidelines can be based on basic health hazard information, statutory or recommended exposure limits set by various agencies, or animal studies. Pels, TLV, Twas, stels and C values of air pollutants (explained below). When evaluating values, do not consider them to be the magic dividing line between "safe" and "unsafe" exposures. In addition, air must be sampled to determine these values in the air you breathe. For most chemicals, this is not a trivial measurement. Usually in SDS, you will see the statement of "data unavailable" in the toxicology information section. Don't think this is equivalent to "safety"; It simply means that the substance has not been evaluated against this value.
Lethal dose refers to the measurement of adverse effects in the test population of a specific animal species. Lethal dose, 50% (LD50) is the calculated single dose (milligrams of substance per kilogram of body weight). When any roe other than inhalation is used, it is expected to lead to mortality in 50% of the test population. When inhaled, the dose is 50% (LC50). It is the concentration of a chemical substance in the breathing air. The calculation result is that the mortality of the test population exposed to the air in a specific time is 50%.
The Occupational Safety and Health Administration (OSHA) has established allowable exposure limits (pels), which are considered to be the maximum concentration of toxic substances inhaled by adult workers 8 hours a day and 40 hours a week without causing injury. Estoppel is a legal limitation.
Threshold Limit Value (TLVs) were determined by the American Conference of government industrial hygiene personnel (ACGIH). TLV is a voluntary and recommended restriction, and TLVs may be different from pels. Most authorities note that TLVs are more reliable than pels because they are revised annually and pel lists are rarely revised.
Time Weighted Average (TWA) is the actual average of worker exposure measured and averaged over an 8-hour working day.
The Short-Term Exposure Limit (STEL) refers to the concentration that should not exceed the short-term (usually 15 minutes) within 8-hour working days, in parts per million or milligrams per cubic meter.
C value refers to some chemicals with serious health hazards. The limit value is a value that cannot be exceeded at any time regardless of the duration.
Summary
All chemicals have inherent hazard characteristics. OSHA has been consistent with American regulations. GHS is used to divide chemical hazards into three categories: physical, health and environmental hazards. In the broad classification of hazards, there are several main subcategories: toxicity, flammability, corrosivity and reactivity. The risks involved in the use of chemicals may range from very low to very high, but if hazards are found and control measures are taken, they can be controlled.
Generally, the purpose of introductory and organic chemistry experiments is to manage known hazards. Under the guidance of the instructor, pay attention to the precautions described on the label, and you will be able to work safely in these laboratories. As you progress in chemistry, you will need to learn how to manage greater hazards by evaluating safety data sheets (SDSS) and using hazard analysis and risk management tools to deepen your understanding of the nature and toxicology of hazardous chemicals
When dealing with chemicals in the laboratory, always carefully avoid or minimize chemical exposure. By understanding the properties of hazardous chemicals, you are adhering to establish best practices, reading safety measures, wearing personal protective equipment (PPE) instructions during the experiment (see Chapter 2), and using volatile organic solvent masks to prevent inhalation - the most common route of exposure (ROE) poisons from entering the body.
Broadly speaking, there are four types of exposure effect relationships:
1) Immediate acute reaction caused by acute exposure (e.g. cyanide poisoning);
2) Acute exposure leads to delayed acute effects (e.g., acetaminophen overdose, initially asymptomatic, liver failure 24-48 hours after acute intake);
3) Acute exposure leads to chronic effects (for example, acute exposure to certain neurotoxic pesticides can lead to chronic neurodegenerative diseases, such as Parkinson's disease);
4) Chronic exposure leads to chronic effects (e.g. alcoholic cirrhosis).
You should realize that as a beginner of chemistry, your understanding of chemical hazards is limited. You are currently in the stage of learning a subject you don't know what you don't know. To protect yourself and others, if you are unsure, ask questions and read all safety information so that you can begin to recognize the dangers associated with the chemicals you use.
Finally, you should see chemicals as a useful tool for chemists and society as a whole. In fact, chemicals are saving lives and improving the quality of life of millions of people every day. Many things we do every day need and use chemicals. We can and must learn to minimize the risk of chemical hazards in order to maintain the safety of ourselves and others.
Reference
5UnitedNations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Fifth revised edition, ST/SG/AC.10/30/Rev. 5; New York andGeneva, 2013.
6OSHA. Hazard CommunicationStandard: Safety Data Sheets, 2012. www.osha. gov/Publications/OSHA3514. html(accessed March 6, 2017).




